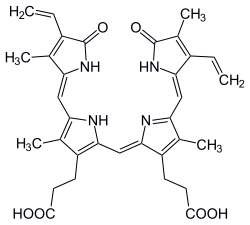
Phycoerythrobilin
 (3E)-phycoerythrobilin
| |
| Names | |
|---|---|
| IUPAC name
(2R,3E,16R)-18-ethenyl-3-ethylidene-1,2,3,15,16,19,22,24-octahydro-2,7,13,17-tetramethyl-1,19-dioxo-21H-biline-8,12-dipropanoic Acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | phycoerythrobilin |
PubChem CID
|
|
| |
| |
| Properties | |
| C33H38N4O6 | |
| Molar mass | 586.689 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phycoerythrobilin is a red phycobilin, i.e. an open tetrapyrrole chromophore[1] found in cyanobacteria and in the chloroplasts of red algae, glaucophytes and some cryptomonads. Phycoerythrobilin is present in the phycobiliprotein phycoerythrin, of which it is the terminal acceptor of energy. The amount of phycoerythrobilin in phycoerythrins varies, depending on the organism. In some Rhodophytes and oceanic cyanobacteria, phycoerythrobilin is also present in the phycocyanin, then termed R-phycocyanin. Like all phycobilins, phycoerythrobilin is covalently linked to these phycobiliproteins by a thioether bond.
Chemical structure
[edit]Phycoerythrobilin is a tetrapyrrole formed in nature by the catabolism of heme B via biliverdin.[2] Two geometric isomers of the compound have been reported, which differ only in the configuration of one sidechain.[3] The stereochemistry of the compounds were confirmed by total synthesis of their methyl esters.[4]
Biosynthesis
[edit](E) and (Z)-phycoerythrobilin are products of porphyrin biosynthesis beyond heme B. They arise from its oxidation which cleaves the macrocyclic ring, giving biliverdin:[2]
Biliverdin is then reduced to give either the (E) or (Z) isomers of phycoerythrobilin, for example by phycoerythrobilin synthase:[2][5][6]
There is evidence that in some organisms it is the (3Z) form that is produced first and that this is converted into the (3E) isomer.[7]
Biological role
[edit]Phycoerythrobilins are components of phycobilisome protein complexes, the light-harvesting antennae that transmit the energy of photons to photosystem II and photosystem I in cyanobacteria and in the chloroplasts of red algae and glaucophytes.[8][9] The absorption spectrum of phycoerythrobilins in the 500–650 nm range where chlorophyll absorbs poorly allows organisms such as Galdieria sulphuraria which use them to be more efficient.[10]
The tetrapyrrole is covalently attached to the phycobiliprotein through a bond between the sulfur of a cysteine amino-acid and the (3E) or (3Z) ring-C=CCH3 sidechain, as a thioether.[7][11]
Uses
[edit]Phycoerythrobilins, in the form of phycobiliproteins, are widely used in foodstuffs and cosmetics as colourants. They are mainly obtained from Spirulina species.[12]
References
[edit]- ^ Chapman, David J.; Cole, W. J.; Siegelman, Harold W. (1967). "Structure of phycoerythrobilin". Journal of the American Chemical Society. 89 (23): 5976–5977. Bibcode:1967JAChS..89.5976C. doi:10.1021/ja00999a058. ISSN 0002-7863.
- ^ a b c Neilan, Brett; Passarini, Michel Rodrigo Zambrano; Singh, Prashant Kumar; Kumar, Ajay, eds. (2023). Cyanobacterial Biotechnology in the 21st Century. Springer Nature. pp. 89–90. doi:10.1007/978-981-99-0181-4. ISBN 978-981-99-0180-7.
- ^ Compare PubChem entry for (3E) isomer with entry for (3Z) isomer
- ^ Gossauer, A.; Weller, J. P. (1978). "Synthesis of bile pigments. 9. Chemical total synthesis of (+)-(2R,16R)- and (+)-(2S,16R)-phycoerythrobilin dimethyl ester". Journal of the American Chemical Society. 100 (18): 5928–5933. doi:10.1021/ja00486a053.
- ^ Dammeyer, Thorben; Bagby, Sarah C.; Sullivan, Matthew B.; Chisholm, Sallie W.; Frankenberg-Dinkel, Nicole (2008). "Efficient Phage-Mediated Pigment Biosynthesis in Oceanic Cyanobacteria". Current Biology. 18 (6): 442–448. doi:10.1016/j.cub.2008.02.067. PMID 18356052.
- ^ Dammeyer, Thorben; Hofmann, Eckhard; Frankenberg-Dinkel, Nicole (2008). "Phycoerythrobilin Synthase (PebS) of a Marine Virus". Journal of Biological Chemistry. 283 (41): 27547–27554. doi:10.1074/jbc.M803765200. PMID 18662988.
- ^ a b Beale, S.I.; Cornejo, J. (1991). "Biosynthesis of phycobilins. 3(Z)-phycoerythrobilin and 3(Z)-phycocyanobilin are intermediates in the formation of 3(E)-phycocyanobilin from biliverdin IX alpha". Journal of Biological Chemistry. 266 (33): 22333–22340. doi:10.1016/S0021-9258(18)54576-2. PMID 1939256.
- ^ Kawakami K, Hamaguchi T, Hirose Y, Kosumi D, Miyata M, Kamiya N, Yonekura K (2022). "Core and rod structures of a thermophilic cyanobacterial light-harvesting phycobilisome". Nature Communications. 13 (1) 3389: 3389. Bibcode:2022NatCo..13.3389K. doi:10.1038/s41467-022-30962-9. PMC 9205905. PMID 35715389.
- ^ Chang L, Liu X, Li Y, Liu CC, Yang F, Zhao J, Sui SF (2015). "Structural organization of an intact phycobilisome and its association with photosystem II". Cell Research. 25 (6): 726–737. doi:10.1038/cr.2015.59. PMC 4456626. PMID 25998682.
- ^ Frascogna, Federica; Rockwell, Nathan C.; Hartmann, Jana; Mudler, Julie M.; Frankenberg-Dinkel, Nicole (2026). "Phycocyanobilin biosynthesis in Galdieria sulphuraria requires isomerization of phycoerythrobilin synthesized by bilin reductases". The FEBS Journal febs.70391. doi:10.1111/febs.70391.
- ^ Sepúlveda-Ugarte, José; Brunet, Juan E.; Matamala, Adelio R.; Martínez-Oyanedel, José; Bunster, Marta (2011). "Spectroscopic parameters of phycoerythrobilin and phycourobilin on phycoerythrin from Gracilaria chilensis" (PDF). Journal of Photochemistry and Photobiology A: Chemistry. 219 (2–3): 211–216. doi:10.1016/j.jphotochem.2011.02.012.
- ^ Ji, Liang; Qiu, Sheng; Wang, Zhiheng; Zhao, Chenni; Tang, Bo; Gao, Zhengquan; Fan, Jianhua (2023). "Phycobiliproteins from algae: Current updates in sustainable production and applications in food and health". Food Research International. 167 112737. doi:10.1016/j.foodres.2023.112737. PMID 37087221.